Tool suite hastens medical-device development
November 06, 2017

LDRA tools help developers comply with IEC 62304
Continuing down the path of software safety, security, and compliance management, LDRA is helping developers in the Class II and Class III medical device space. The goal is to create safe and effective medical devices faster, and at a lower cost, while mitigating risk. The latest LDRA tools do this through:
- Compliance management for IEC 62304
- Lifecycle traceability, from functional, safety, and security requirements throughout the software development lifecycle
- Static code analysis, analyzing software for various quality metrics such as clarity, testability
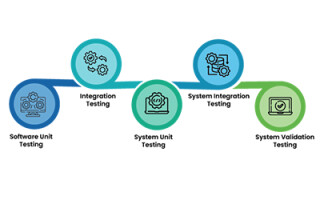
- Automated unit and system level testing, automatically generating test cases, test harnesses, and automatically executing those test cases
- Providing the ability to measure the effectiveness of testing processes through structural coverage analysis, and graphically showing what code has and hasn’t been tested.
According to LDRA, they're automating many of the tasks that were previously done manually. This includes automated software quality analysis and testing, while adhering to IEC62304.
In a nutshell, the tool suite improves productivity and efficiency through automation while mitigating risk; performs a software quality analysis; ensures code consistency; and automates the process of test case generation, test harness generation, test execution, and structural coverage analysis.
The LDRA tool suite for Medical Devices is available now.